In the pathway of biopharmaceutical innovation, preclinical studies serve as a cornerstone for translational success. At Prisys Biotech, we are dedicated to advancing nonhuman primate (NHP) translational research, providing pharmaceutical companies and academic institutions worldwide with high-quality preclinical support.
With extensive expertise in bleeding/AV shunt and MCAO/ICH models, our team has successfully established a robust portfolio of NHP models that deliver high translational value for hematology and cerebrovascular drug development.
AV Shunt & Hemophilia Models: A Reliable Platform for Thrombosis and Hemostasis Research
In hematology and anticoagulant drug development, Prisys Biotech has established multiple NHP models, including those for anemia, bleeding, coagulation, and thrombosis. Among them, the arteriovenous (AV) shunt thrombosis model is particularly distinguished and widely applied in the pharmacodynamic evaluation of antithrombotic therapies.
Technical Strengths
- 15+ years of surgical expertise in NHP vascular procedures ensures precise and reproducible AV shunt model establishment.
- Operational consistency guarantees robust and reliable experimental data.
- IND-enabling experience, ensuring compliance with regulatory requirements for new drug development.
Applications
- Hemostasis and antithrombotic drug evaluation
- Hemophilia research and novel therapy validation
- Intracerebral hemorrhage and hemorrhagic shock studies
- Wound healing and regenerative medicine
Representative Findings
In the cynomolgus monkey AV shunt model, the FXI activation-blocking antibody BJTJ-1837 demonstrated strong antithrombotic efficacy without increasing bleeding risk【1】.

In hemophilia monkey models, Type II antibodies showed dose-dependent efficacy in both acute and prophylactic settings, selectively inhibiting APC's anticoagulant function without impairing cytoprotective pathways【2】.

MCAO & ICH Models: Advanced Tools for Stroke Research
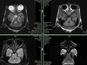
Ischemic stroke and intracerebral hemorrhage (ICH) remain among the leading causes of mortality and disability worldwide. Prisys Biotech has developed digital subtraction angiography (DSA)-guided MCAO and ICH models, closely replicating clinical stroke pathophysiology.
Model Highlights
DSA-guided autologous thrombus injection into the middle cerebral artery (MCA), accurately mimicking clinical thrombus formation【3】.

Minimally invasive, reproducible, and precise, with low complication rates and reduced mortality.
Multi-Modal Imaging Evaluation
- Comprehensive monitoring via CT/CTA/CTP and MRI (DWI, ADC, SWI, T2WI, FLAIR, MRA).
- Real-time evaluation of thrombus formation, reperfusion, and infarct progression.
- Imaging data supports both lesion assessment and therapeutic efficacy prediction.
Pharmacological and Pathological Validation
Enables preclinical efficacy studies of thrombolytic agents such as rt-PA, replicating clinical treatment workflows.
TTC staining and MRI correlation confirm infarct volume reduction following thrombolysis.
Precision CNS Drug Delivery: Overcoming the Blood–Brain Barrier
Leveraging Asia's first intraoperative MRI (iMRI) navigation system for NHPs, combined with convection-enhanced delivery (CED), Prisys Biotech has built a state-of-the-art CNS drug delivery platform.

Key Advantages
- Direct BBB penetration for efficient targeted delivery.
- Sub-millimeter precision with intraoperative trajectory optimization.
- Real-time visualization of drug distribution, facilitating translational success into clinical trials.
- Integration of MRI, CT, DSA, and ultrasound imaging, providing a systematic solution for precise CNS delivery.
Conclusion
Prisys Biotech has established a comprehensive suite of NHP hematology and vascular disease models, including:
- Bleeding and thrombosis models (AV shunt, hemophilia)
- Ischemic and hemorrhagic stroke (MCAO, ICH)
- Embolic myocardial infarction
- Anemia and hematopoiesis studies
- Metabolic vascular diseases (e.g., pulmonary hypertension)
With advanced hematology assays (coagulation, blood gas analysis, dynamic blood pressure, catheter-based monitoring) and precision CNS delivery technologies, Prisys provides pharmaceutical partners with reliable, clinically relevant platforms for preclinical research.
👉 Contact us to learn more:
🌐 www.prisysbiotech.com
📧 bd@prisysbiotech.com | ☎ +86-21-50435616
References
He X, Zhang J, Du Y, et al. BJTJ-1837, a novel FXI activation-blocking antibody. Res Pract Thromb Haemost. 2023;7(2):100067.
Zhao XY, Wilmen A, Wang D, et al. Targeted inhibition of activated protein C by a non-active-site inhibitory antibody to treat hemophilia. Nat Commun. 2020;11:2992.
Ye J, Shang H, Du H, et al. An optimal ischemic stroke model established by DSA-guided autologous thrombi in cynomolgus monkeys. Front Neurol. 2022;13:864954.